The development of chimeric antigen receptor (CAR)-T cell therapy has revolutionized cancer treatment, particularly for hematologic malignancies. However, its long-term efficacy in solid tumors, such as neuroblastoma, remains a significant area of research. One of the earliest clinical trials to explore GD2-directed CAR-T therapy in neuroblastoma patients has provided valuable insights, with follow-up data now extending up to 18 years. This article explores the long-term outcomes of these pioneering efforts and what they mean for the future of CAR-T cell therapy in pediatric oncology.
Understanding GD2-Directed CAR-T Cell Therapy
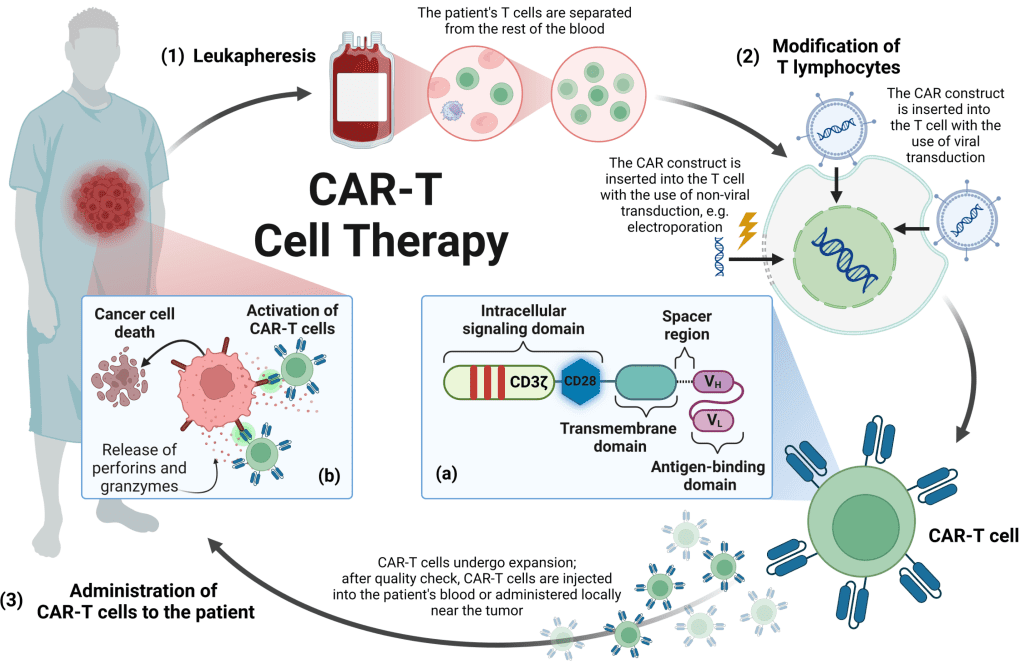
Neuroblastoma is one of the most aggressive pediatric cancers, and despite advances in treatment, relapsed or refractory cases remain challenging. GD2, a surface antigen highly expressed in neuroblastoma cells, has been a prime target for immunotherapy.
CAR-T cell therapy involves genetically modifying a patient’s T cells to recognize and attack cancer cells expressing specific antigens, such as GD2. While this approach has been successful in blood cancers, its application in solid tumors presents unique obstacles, including the tumor microenvironment, limited persistence of CAR-T cells, and the potential for severe toxicity.
The Pioneering Clinical Trial (2004-2009)
Between 2004 and 2009, a groundbreaking phase 1 clinical trial investigated GD2-directed CAR-T therapy in children with neuroblastoma. Two types of immune cells were used:
- Epstein–Barr virus (EBV)-specific T lymphocytes (VSTs)
- CD3-activated T cells (ATCs)
These cells were genetically modified with first-generation CARs lacking co-stimulatory domains, a feature now considered essential in modern CAR designs. Despite this limitation, the trial yielded remarkable long-term responses, with some patients achieving sustained remission for more than a decade.
Video : Revolutionary CAR T Therapy: A New Hope for Neuroblastoma
Key Findings from the 18-Year Follow-Up
Researchers tracked 19 patients over nearly two decades, monitoring their response to CAR-T therapy. The findings revealed crucial insights into the long-term impact of this treatment.
1. Long-Term Remission in Some Patients
Among 11 patients who had active disease at the time of infusion, three achieved complete remission. Two of these patients remained in sustained remission—one for eight years before being lost to follow-up and another for more than 18 years. This suggests that even early-generation CAR-T cells, without co-stimulatory domains, can lead to durable responses in select cases.
2. High Disease-Free Survival in Patients with No Evidence of Disease at Infusion
Eight patients who had no active disease at the time of CAR-T administration were followed closely. Among them, five remained disease-free for 10-15 years, reinforcing the potential role of CAR-T cells as a consolidation therapy after achieving initial remission through conventional treatments.
3. Prolonged CAR-T Cell Persistence
One of the critical determinants of CAR-T cell efficacy is how long the engineered T cells persist in the body. This study found that transgene signals—indicating the presence of CAR-T cells—were intermittently detectable for years after infusion. Patients who had long-term remission exhibited greater CAR-T cell persistence, suggesting that sustained immune surveillance might play a crucial role in preventing relapse.
4. Unexpected Success Despite First-Generation CAR-T Cells
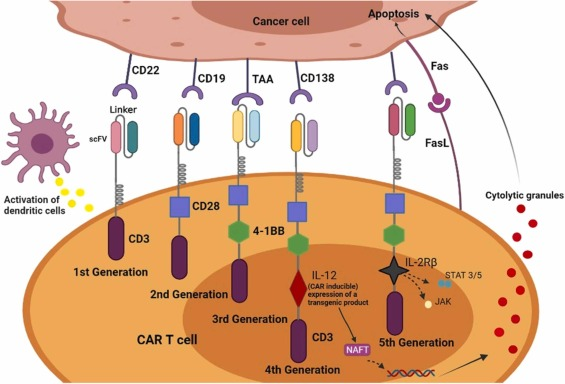
Modern CAR-T cell therapies incorporate co-stimulatory domains, such as CD28 or 4-1BB, which enhance cell persistence and function. However, this early trial used first-generation CARs, which lacked these enhancements. The fact that some patients still achieved long-term remission challenges previous assumptions about the necessity of co-stimulatory domains in every case.
5. No Evidence of T-Cell Malignancies
A recent concern in CAR-T therapy is the potential for secondary malignancies arising from genetically modified T cells. The FDA has reported rare cases of T-cell lymphoma associated with CAR-T therapy in other clinical settings. However, in this 18-year follow-up, no cases of secondary T-cell malignancies were observed, providing reassurance about the long-term safety of this approach.
Implications for Future CAR-T Cell Therapies in Solid Tumors
While CAR-T therapy has transformed the treatment of hematologic malignancies like leukemia and lymphoma, its success in solid tumors has been more limited. This study provides valuable lessons for improving CAR-T cell therapy for neuroblastoma and other solid cancers.
1. The Role of Persistence in CAR-T Efficacy
Long-term persistence of CAR-T cells correlated with better outcomes in this study. Enhancing T-cell longevity remains a critical goal for future CAR designs. Second- and third-generation CARs with optimized co-stimulatory domains may help achieve even greater durability and effectiveness.
Video : New clinical trial of boosted CAR T-cells for neuroblastoma
2. Importance of Tumor Burden at the Time of Infusion
Patients who had no detectable disease at the time of infusion had a higher rate of long-term survival. This suggests that CAR-T therapy might be most effective as a consolidation treatment following chemotherapy, surgery, or radiation, rather than as a standalone therapy for active disease.
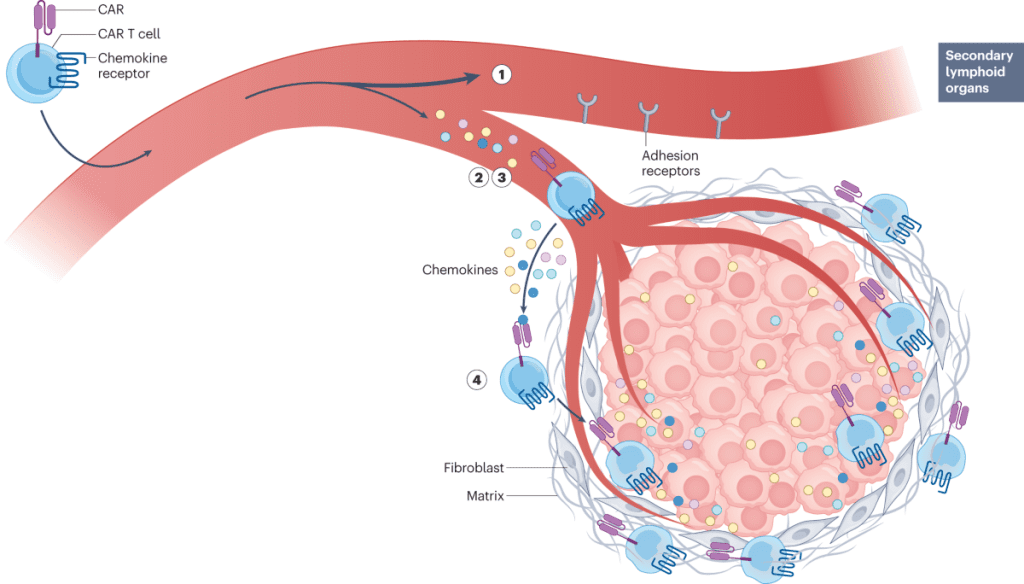
3. The Need for Improved Tumor Microenvironment Targeting
One of the primary challenges in treating solid tumors with CAR-T cells is overcoming the immunosuppressive tumor microenvironment. Future strategies may include combining CAR-T therapy with immune checkpoint inhibitors or using gene-editing techniques to enhance T-cell infiltration into tumors.
4. Potential for Combination Approaches
Given that some patients achieved long-term remission while others did not, combining CAR-T therapy with additional immunotherapies, such as monoclonal antibodies or cytokine-based therapies, may improve overall response rates.
Challenges and Limitations of CAR-T Therapy in Neuroblastoma
Despite the promising findings, significant hurdles remain in the widespread adoption of CAR-T therapy for neuroblastoma.
- High Variability in Patient Response – While some patients experienced complete remission, others had only transient benefits.
- Potential for Toxicity – GD2-targeted therapies can lead to severe side effects, such as neuropathic pain, due to GD2 expression in normal nerve tissues.
- Cost and Accessibility – CAR-T therapies remain expensive and are not yet widely available for pediatric solid tumors.
Conclusion: A Milestone in CAR-T Therapy for Solid Tumors
The 18-year follow-up of this early-phase trial offers a rare glimpse into the long-term potential of GD2-directed CAR-T therapy in neuroblastoma. The findings demonstrate that even first-generation CAR-T cells can lead to prolonged remission in some patients, challenging previous assumptions about their effectiveness.
While modern CAR-T therapies have evolved significantly, this study underscores the need for continued research into optimizing CAR persistence, improving tumor microenvironment targeting, and refining combination strategies. As CAR-T therapy continues to advance, its role in treating solid tumors like neuroblastoma may become even more promising.
These long-term results are a testament to the potential of CAR-T cell therapy and offer hope for pediatric cancer patients facing relapsed or refractory neuroblastoma. With ongoing innovations in the field, the future of immunotherapy in solid tumors is brighter than ever.


