What if we could train the body to prevent cancer just like we train it to fight off viruses? That’s exactly what researchers are aiming for with BNT116, the world’s first mRNA-based lung cancer vaccine, now entering clinical trials in seven countries. Developed by BioNTech, the same biotech powerhouse behind one of the first COVID-19 vaccines, this groundbreaking project could completely change how we fight lung cancer—shifting the model from reactive treatment to proactive prevention.
Let’s unpack what BNT116 is, how it works, and why it has the potential to revolutionize the future of oncology.
Targeting Non-Small Cell Lung Cancer with Precision
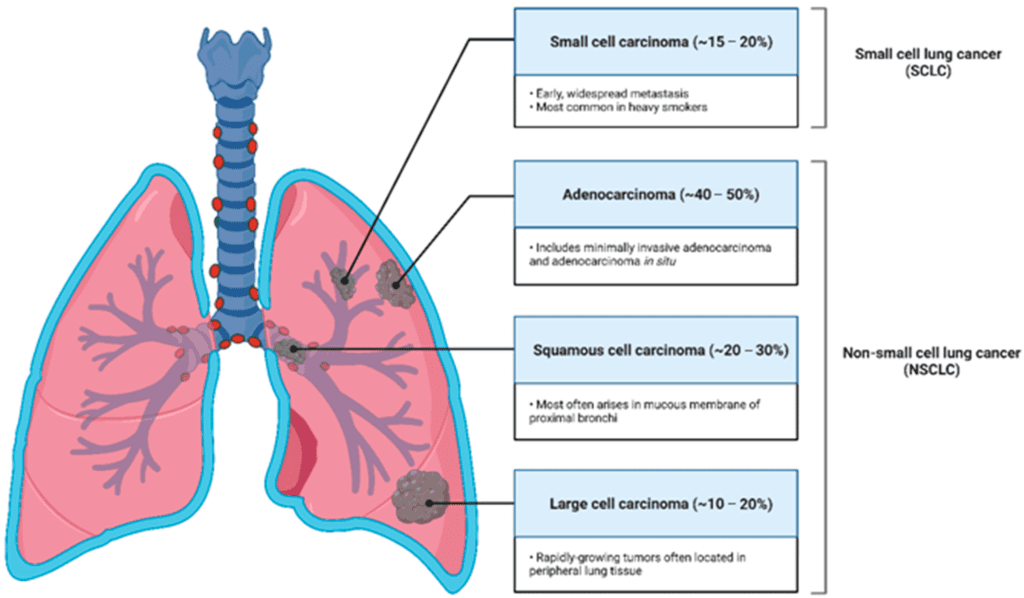
BNT116 was designed specifically to target non-small cell lung cancer (NSCLC)—a type that accounts for around 85% of all lung cancer cases worldwide. It’s a disease known for being aggressive, hard to catch early, and stubbornly resistant to conventional therapies.
The unique part about BNT116 is that it uses mRNA technology—the same platform used in modern COVID-19 vaccines—to “teach” the immune system how to recognize and destroy cancer cells. But instead of targeting viruses, this vaccine presents cancer-specific antigens to the body’s defense system, allowing it to hunt down and destroy malignant cells with laser precision.
Inside the Science: How mRNA Cancer Vaccines Work
So how does it all work? The science is brilliant in its simplicity.
BNT116 delivers synthetic messenger RNA to the body—tiny strands of genetic code that instruct cells to produce specific proteins associated with cancer. Once the immune system detects these abnormal proteins, it flags them as a threat and kicks into action. This mechanism mimics how traditional vaccines work but is customized for tumor antigens, effectively marking cancer for destruction.
The goal? To generate a strong, targeted immune response that not only destroys existing cancer cells but also prevents the cancer from coming back. That’s the real magic of mRNA technology—it’s fast, adaptable, and highly programmable.
Video : 🫁The world’s first mRNA vaccine trial for lung cancer has begun!
Where the Trials Are Happening: A Global Effort
The Phase 1 clinical trial for BNT116 is currently underway in seven countries:
- United States
- United Kingdom
- Germany
- Hungary
- Poland
- Spain
- Turkey
Around 130 patients with advanced non-small cell lung cancer are participating across 34 clinical sites. Researchers are currently evaluating the vaccine’s safety, tolerability, and immune activation, with the ultimate goal of determining whether it can effectively reduce tumor progression and boost long-term survival.
Why so many countries? Because cancer knows no borders—and the fight against it shouldn’t either.
Why This Could Be a Game-Changer for Cancer Treatment
Traditional lung cancer treatments like chemotherapy and radiation are powerful, but they come at a cost—harsh side effects, non-specific targeting, and, in many cases, limited long-term success. What makes BNT116 different is that it’s designed to work with the body, not against it.
Instead of carpet-bombing healthy and cancerous cells alike, this vaccine turns the immune system into a highly selective assassin, hunting only the malignant targets. That opens the door to fewer side effects, better outcomes, and the potential to prevent relapses, which are notoriously common in lung cancer.
This isn’t just a new treatment. It’s a paradigm shift—from fighting cancer after it appears to preparing the body in advance to eliminate it on sight.
What Sets BNT116 Apart from Other Therapies
You might be wondering, aren’t there already immunotherapies for lung cancer? Yes—but BNT116 is different in several key ways:
- It’s personalized. The mRNA sequence can be tailored to target specific mutations or proteins found in a patient’s tumor.
- It’s fast to produce. Once the tumor markers are identified, new vaccine batches can be created in weeks, not months.
- It may reduce recurrence. By priming the immune system, it could help prevent the cancer from returning after remission.
While checkpoint inhibitors and targeted drugs have made significant strides, they still fall short in many patients. BNT116 could potentially fill that gap.
The Road Ahead: Hope Meets Caution
Right now, we’re still in the early stages. Phase 1 is about laying the foundation—ensuring that the vaccine is safe and effective in small groups before moving on to larger populations.
Video : World first mRNA lung cancer vaccine trial begins, offering hope for thousands
If successful, BNT116 could progress to Phase 2 and 3 trials, where researchers will further measure its effectiveness against placebos or standard care. That means we’re still a few years away from widespread availability, but the signs are extremely promising.
BioNTech also aims to expand the technology by combining it with checkpoint inhibitors, another type of immunotherapy, to maximize immune activation against resistant tumors.
Conclusion: A New Chapter in the War on Cancer
BNT116 isn’t just a vaccine—it’s a potential lifesaver for millions of people around the world. With over 1.8 million deaths attributed to lung cancer each year, the need for smarter, more precise therapies has never been more urgent.
If successful, this could usher in a new era of personalized cancer medicine—one where we don’t wait for cancer to strike, but equip the body to stop it in its tracks before it spreads. Thanks to BioNTech’s relentless innovation, the world might soon have a proactive weapon against one of humanity’s deadliest diseases.
And that’s not just progress. That’s hope.


