What if your cells had a built-in countdown to death? As eerie as that sounds, new research has unveiled just that—a mortality timer within our cells, determined by the size of the nucleolus. It turns out, the nucleolus isn’t just a little blob inside the nucleus that manages ribosomal RNA—it might be quietly dictating how long your cells live.
Let’s break down this breakthrough in plain English, explore why nucleolar size matters, and what it means for aging, disease, and potentially, longevity itself.
Nucleolar Size and Aging: The Connection Scientists Can No Longer Ignore
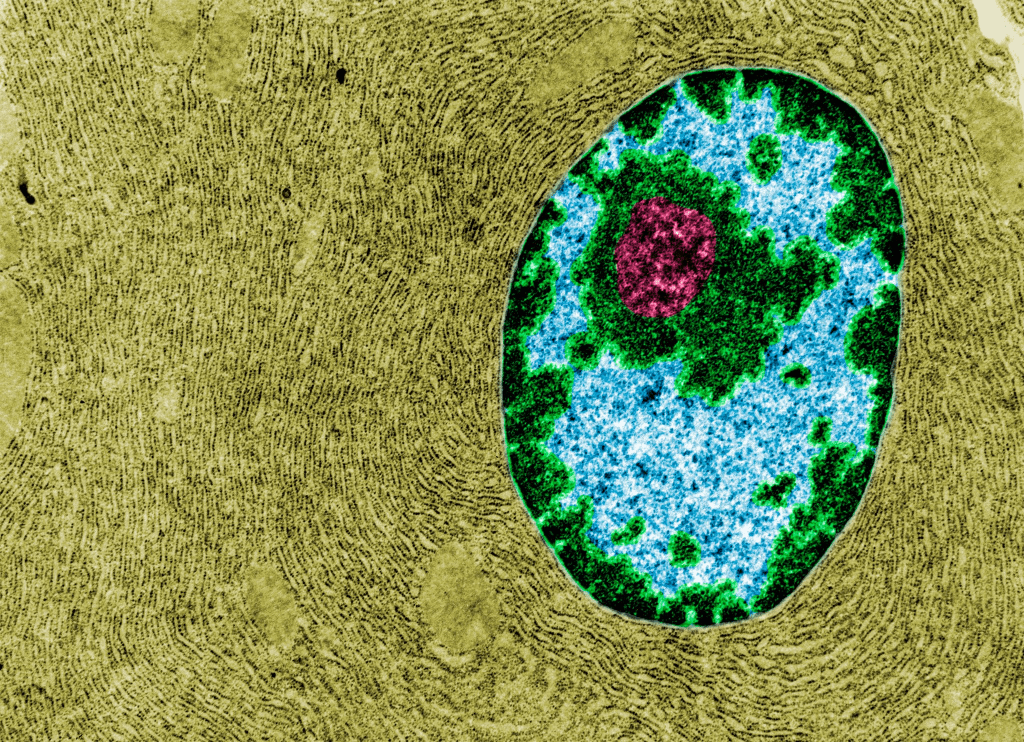
We already know that genome instability is a core hallmark of aging. One of the most vulnerable areas in the genome is the ribosomal DNA (rDNA) housed within the nucleolus—a dense region inside the cell’s nucleus. Interestingly, researchers have observed that as organisms age, from yeast to humans, their nucleoli expand. On the flip side, longevity-boosting strategies like calorie restriction and certain drugs often result in smaller nucleoli.
Coincidence? Not even close.
A new study reveals that nucleolar expansion isn’t just a symptom of aging—it’s actually a driver of the process. The team of scientists behind the study engineered yeast to artificially shrink their nucleoli. The result? These cells lived significantly longer. And no, this didn’t happen because of changes to protein synthesis or silencing of rDNA—two things usually tied to nucleolar function. It happened because of something far simpler but far more powerful: nucleolar integrity.
How Oversized Nucleoli Break the Rules—and Your DNA
Think of the nucleolus like an exclusive VIP club. Only certain molecules are allowed in. As long as the club maintains tight control over who enters, everything stays in order. But once the nucleolus expands past a certain size, it starts to lose that selective barrier. It becomes leaky.
Video : Control of Transient Genomic Instability / Cell, August 9, 2018 (Vol. 174, iss. 4)
When this happens, unwanted guests like Rad52—a protein involved in DNA repair—start slipping inside. Once inside, Rad52 kicks off abnormal recombination events in the rDNA. This leads to a cascade of DNA damage, genomic instability, and eventually cell death.
This is how nucleolar expansion turns into a death sentence. It’s not just crowding the nucleus—it’s unleashing chaos inside your genome.
A Built-In Mortality Timer: Your Nucleolus Knows When It’s Time to Go
Here’s where things get wild. Researchers believe the nucleolus acts like a cellular timer, counting down a cell’s remaining lifespan based on its size. Once the nucleolus crosses a critical threshold in volume, the structure breaks down, permeability goes haywire, and the cell spirals toward irreversible genome damage.
This changes the game for how we think about cellular aging. It’s not just about oxidative stress or telomere shortening anymore. The biophysical state of the nucleolus may be one of the most accurate predictors of cellular lifespan we’ve ever seen.
It’s Not Just Yeast—This Applies to Us Too
Before you dismiss this as “just a yeast thing,” remember this: similar nucleolar changes have been observed in humans and other mammals. In fact, nucleolar hypertrophy—an enlarged nucleolus—is frequently seen in age-related diseases like cancer and neurodegeneration.
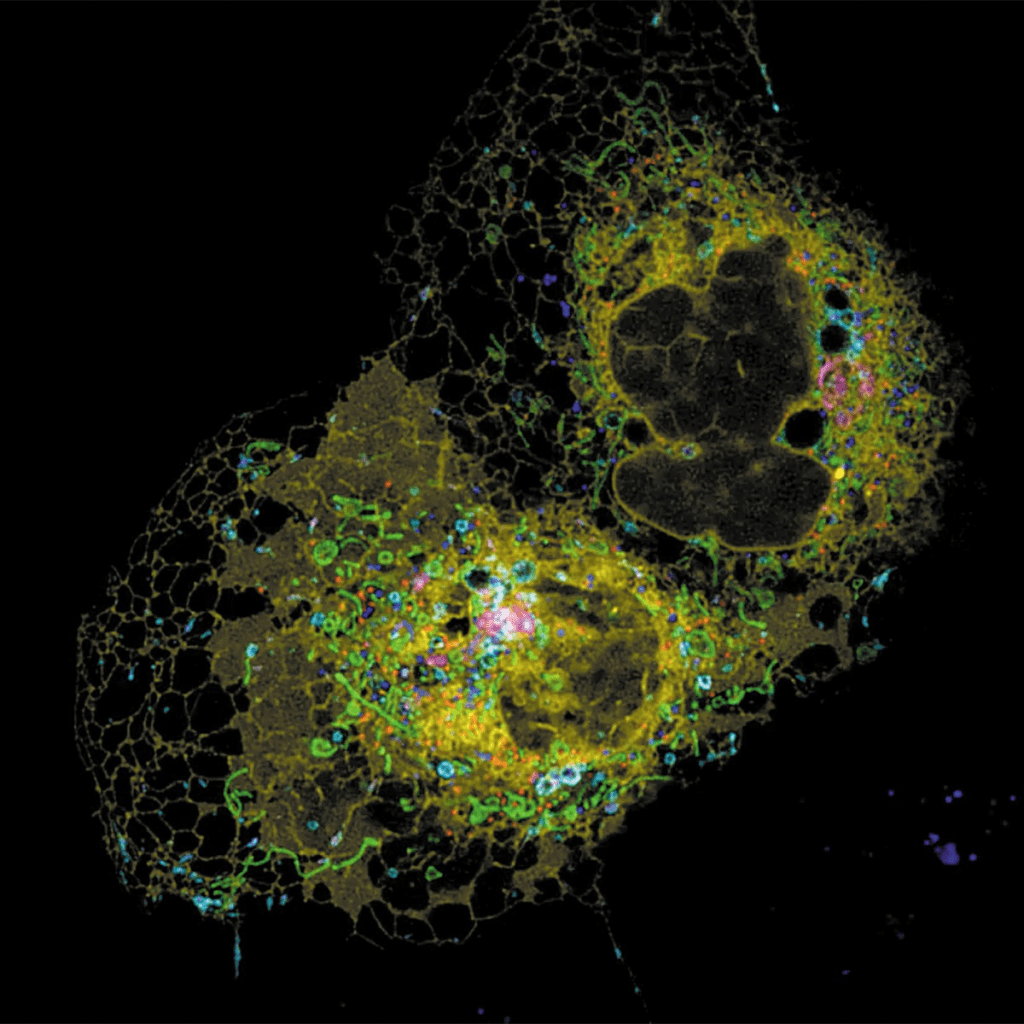
This strongly suggests that what scientists observed in yeast could be at play in human cells too. If your nucleoli are expanding, it might mean your cells are inching closer to instability—and you’re inching closer to age-related decline.
Can We Actually Target Nucleolar Size to Extend Lifespan?
Here’s the million-dollar question: if nucleolar size is a trigger for aging, can we shrink or stabilize it to slow the process down?
The research suggests a few promising approaches:
- Pharmacological compounds that stabilize nucleolar structure and reinforce its selective barrier
- Caloric restriction and metabolic interventions like fasting or the drug rapamycin, which are already shown to reduce nucleolar size and extend lifespan in animals
- Genetic engineering that enhances nucleolar resistance to expansion and maintains compartmental integrity
In short, if we can prevent the nucleolus from reaching its tipping point, we might also prevent the domino effect of DNA damage that leads to cellular death and degeneration.
Why This Discovery Could Reshape the Future of Anti-Aging Science
This isn’t just another molecular biology discovery—it’s a potential paradigm shift. Aging has always been viewed as a gradual, complex process involving dozens of systems wearing down over time. But this study adds a surprisingly simple trigger to that complexity: when the nucleolus grows too large, the cell falls apart.
That means the key to longevity could be hidden not in complicated gene therapies, but in maintaining a stable and compact nucleolus.
Video : Gene Silencing by Micro RNA – Studio Katharina Petsche
It’s a bit like managing clutter in a house. As long as the rooms stay organized, everything works. But once you let things expand beyond their limits, chaos takes over—and the damage becomes harder and harder to reverse.
Conclusion: A New Frontier in the Science of Aging
The idea that a single organelle’s size could dictate lifespan is groundbreaking. The nucleolus, once thought to be a simple ribosome factory, turns out to be a powerful regulator of aging, health, and genome integrity.
So where do we go from here? More research, for sure. But the implications are already huge. If scientists can find a way to control nucleolar expansion, they could potentially extend healthy lifespan, reduce age-related diseases, and even enhance cellular repair.
For now, this discovery gives us something to think about: maybe aging isn’t just about time—it’s about space. And maybe the secret to longevity lies in keeping the nucleolus compact, clean, and closed to unwanted chaos.