Aging is a complex biological process, and one of its most defining characteristics is genome instability. Among the many cellular components that play a role in aging, the nucleolus—the central hub of ribosomal RNA (rRNA) production—has emerged as a key player. Scientists have observed that nucleolar enlargement correlates with aging, from yeast to mammals, raising important questions about its role in cellular lifespan and genomic stability.
Recent research has uncovered a mortality timer linked to nucleolar size, demonstrating that nucleolar integrity loss can lead to catastrophic genomic instability. This finding has profound implications for understanding aging, genome maintenance, and potential anti-aging interventions.
In this article, we will explore how nucleolar expansion contributes to aging, why it triggers genomic instability, and how regulating nucleolar size could be a strategy for extending lifespan.
The Role of the Nucleolus in Cellular Function and Aging
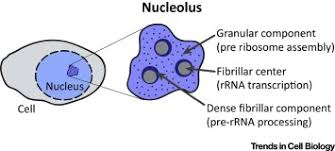
The nucleolus is a specialized structure within the nucleus, primarily responsible for ribosomal RNA (rRNA) synthesis and ribosome assembly. It plays a crucial role in maintaining cellular function by regulating protein synthesis and genome stability.
However, as cells age, the nucleolus undergoes structural changes, including enlargement and increased disorganization. This has been observed in various organisms, from budding yeast to human cells. Scientists have long speculated that these changes contribute to cellular senescence and aging-related diseases, but the underlying mechanisms were not well understood.
Nucleolar Enlargement and Aging: What Happens Inside the Cell?
As cells divide and age, nucleolar expansion is accompanied by:
✔️ Increased ribosomal DNA (rDNA) instability – rDNA contains highly repetitive sequences that are particularly prone to recombination errors.
✔️ Altered protein composition – Normally, the nucleolus excludes certain proteins to maintain its unique biophysical properties. However, expansion disrupts this barrier, allowing inappropriate proteins to enter.
✔️ Genome instability and premature cell death – Once the nucleolus loses its integrity, it sets off a chain reaction of genomic instability, leading to replicative lifespan reduction.
Video : Cognitive Slowness and Decline Linked to Low-Normal B12 Levels
These observations led researchers to hypothesize that nucleolar size might act as a “mortality timer” for the cell—a built-in mechanism that dictates when genomic instability will ultimately lead to cell death.
Experimental Evidence: How Nucleolar Size Controls Lifespan
To directly test whether nucleolar expansion drives aging, scientists developed an engineered system to reduce nucleolar size in budding yeast. This system allowed them to study whether smaller nucleoli could extend cellular lifespan.
Key Findings from the Study
🔬 Reducing nucleolar size significantly extended the replicative lifespan of yeast cells. This effect was independent of changes in protein synthesis rate or rDNA silencing, suggesting that the key factor was nucleolar integrity itself.
🔬 When nucleoli expanded beyond a critical size, their physical properties changed. This change allowed proteins like Rad52, a key homologous recombination repair protein, to enter the nucleolus.
🔬 The entry of Rad52 into the nucleolus triggered rDNA instability, leading to genomic catastrophe. Normally, Rad52 is excluded from the nucleolus to prevent aberrant recombination events in rDNA. However, when nucleolar expansion disrupted this exclusion, it led to rDNA recombination errors, structural damage, and eventual cell death.
These findings established a direct link between nucleolar size and genome stability, proving that nucleolar expansion is not just a consequence of aging, but an active driver of cellular decline.
How Nucleolar Expansion Becomes a Mortality Timer
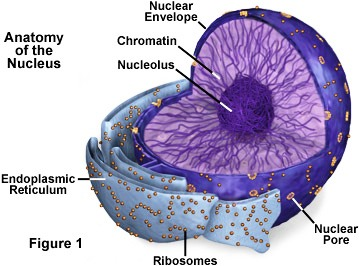
Cells have built-in mechanisms to maintain genomic stability, but once nucleolar expansion crosses a critical threshold, it disrupts these protective systems. This creates a self-reinforcing cycle of instability and DNA damage, ultimately leading to:
✔️ rDNA recombination errors → Structural changes in the genome.
✔️ Loss of nucleolar integrity → Entry of proteins that should remain excluded.
✔️ Accumulation of DNA damage → Triggers stress responses and cell death.
This means that nucleolar size serves as a countdown clock—once it expands beyond a specific threshold, it triggers catastrophic genome instability and cell mortality.
Implications for Aging and Anti-Aging Interventions
The discovery that nucleolar expansion drives aging has far-reaching implications for anti-aging research. If nucleolar enlargement is a key factor in cellular decline, targeting nucleolar integrity could slow down aging and extend lifespan.
Potential Strategies to Control Nucleolar Size
🧬 Dietary and metabolic interventions – Many anti-aging strategies, such as caloric restriction and rapamycin treatment, have been shown to reduce nucleolar size. This suggests that dietary and metabolic interventions may help maintain nucleolar integrity.
🧬 Pharmacological approaches – Developing drugs that regulate nucleolar proteins could help prevent nucleolar enlargement and genome instability.
🧬 Gene regulation therapies – Modulating the expression of key nucleolar components might offer a way to delay aging at the molecular level.
Video : A human cardiomyocyte mitochondrion – 3D Medical Animation
These findings open the door for new approaches to combat age-related diseases by directly targeting the nucleolus.
Final Thoughts: A Breakthrough in Understanding Aging
The nucleolus, once thought to be solely responsible for ribosome production, is now recognized as a critical player in aging and genome stability.
This research redefines the role of nucleolar expansion by showing that it isn’t just a passive marker of aging—it is an active driver of cellular decline. More importantly, by regulating nucleolar size, it may be possible to extend lifespan and delay age-related genomic instability.
As scientists continue to explore new ways to maintain nucleolar integrity, this discovery paves the way for novel anti-aging strategies that could one day revolutionize how we approach aging and longevity.
The future of anti-aging research may be hidden within the nucleolus. Are we on the brink of unlocking the secrets to extending human lifespan? Stay tuned!